Application Areas
- Energy Efficiency Improvement
- Alternative Energy Research
- Environmental Protection
- Process Design, Simulation & Optimization
- Reaction Engineering & Kinetics
- Chemical Process Unit Design & Optimization
- New Drugs Development & Health Researches
- Cosmetics, Flavors & Fragrance Design
- Semiconductor researches
Categorized Chemical Compounds Available

- 384,000Free Radicals
- 958,000Hydrocarbons
- 1,510,000Hetero Compounds
- 50 000Halogen Compounds
- 10,000Extra-hetero Compounds
- 1,312,000Drug-like Compounds
- 105,000Gasoline Compounds
- 171,000Jet-fuel Compounds
- 735,000Diesel Compounds
- 672,000Bio-diesel Compounds

- 248,000Soot Aromatic Compounds
- 273,000Naphtha Compounds
- 1,349,000 Compounds for Combustion Process
- 491,000 Compounds for Thermal Cracking Process
- 408,000 Compounds for Catalytic Reforming Process
- 798,000 Compounds for Catalytic Cracking Process
- 768,000 Compounds for Hydro Cracking Process
- 1,012,000 Compounds for Desulfurization Process
- 231,000 Compounds for Isomerization Process
- 858,000 Compounds for GTL (Gas-To-Liquid) or
- Fischer-Tropsch Process
- 1,249,000 Compounds for CTL (Coal-To-Liquid) Process
- 689,000 Compounds for MTO (Methanol- To-Olefin)
- or MTG (Methanol- To- Gasoline) Process
Available Chemical Groups Including
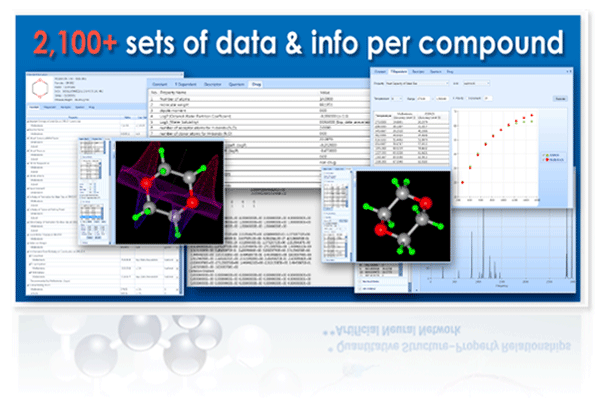
Properties & Information Available for Every Compound
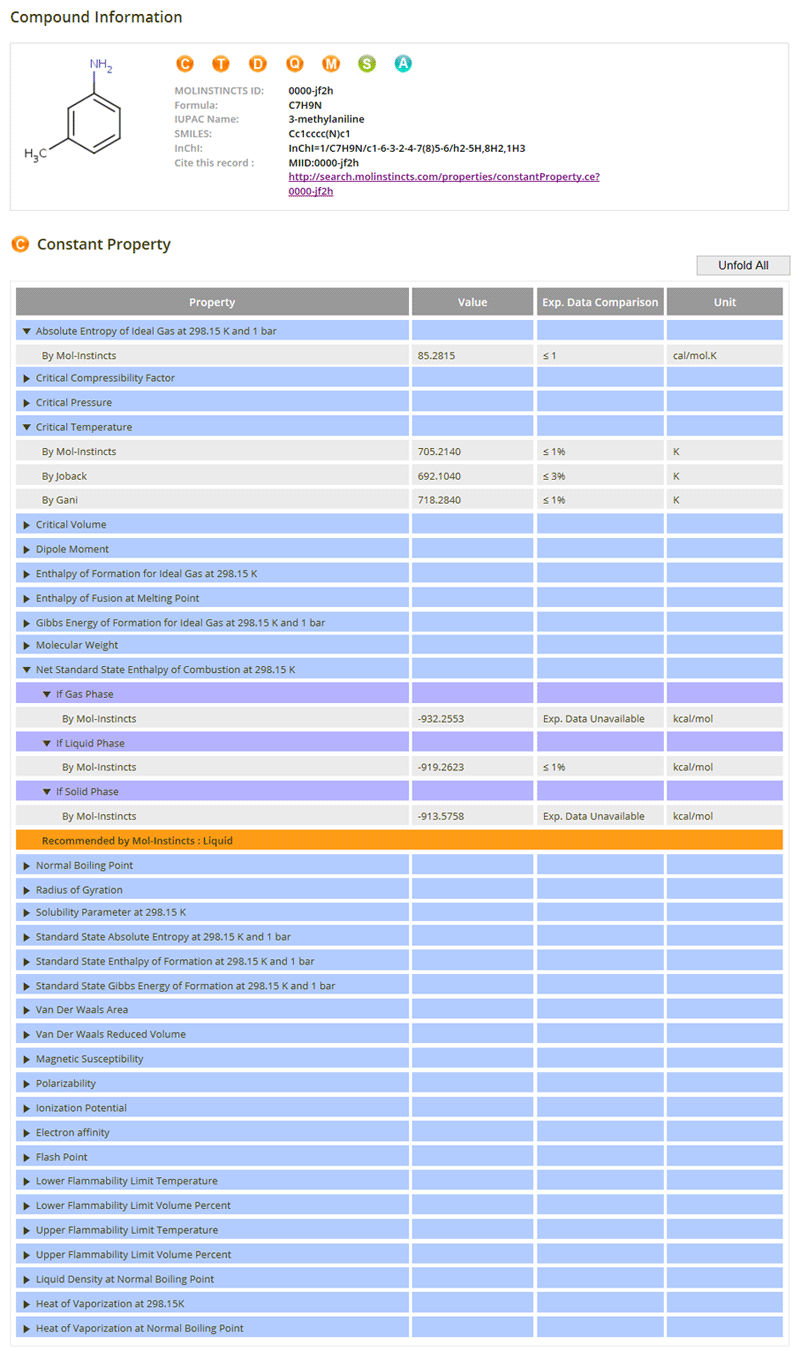
Thermo-Physico-Chemical Properties (constant)
01- Acentric Factor
- Critical Compressibility Factor
- Critical Pressure
- Critical Temperature
- Critical Volume
- Dipole Moment
- Enthalpy of Formation
- Enthalpy of Fusion
- Gibbs Energy of Formation
- Liquid Molar Volume
- Standard State Enthalpy of Combustion
- Normal Boiling Point
- Radius of Gyration
- Refractive Index
- Solubility Parameter
- Standard State Absolute Entropy
- Standard State Enthalpy of Formation
- Standard State Gibbs Energy of Formation
- Van Der Waals Area
- Van Der Waals Reduced Volume
- Magnetic Susceptibility
- Polarizability
- Ionization Potential
- Electron affinity
- Flash Point
- Parachor
- Lower Flammability Limit Temperature
- Lower Flammability Limit Volume Percent
- Upper Flammability Limit Temperature
- Upper Flammability Limit Volume Percent
- Liquid Density at Normal Boiling Point
- Heat of Vaporization at 298.15K
- Heat of Vaporization at Normal Boiling Point
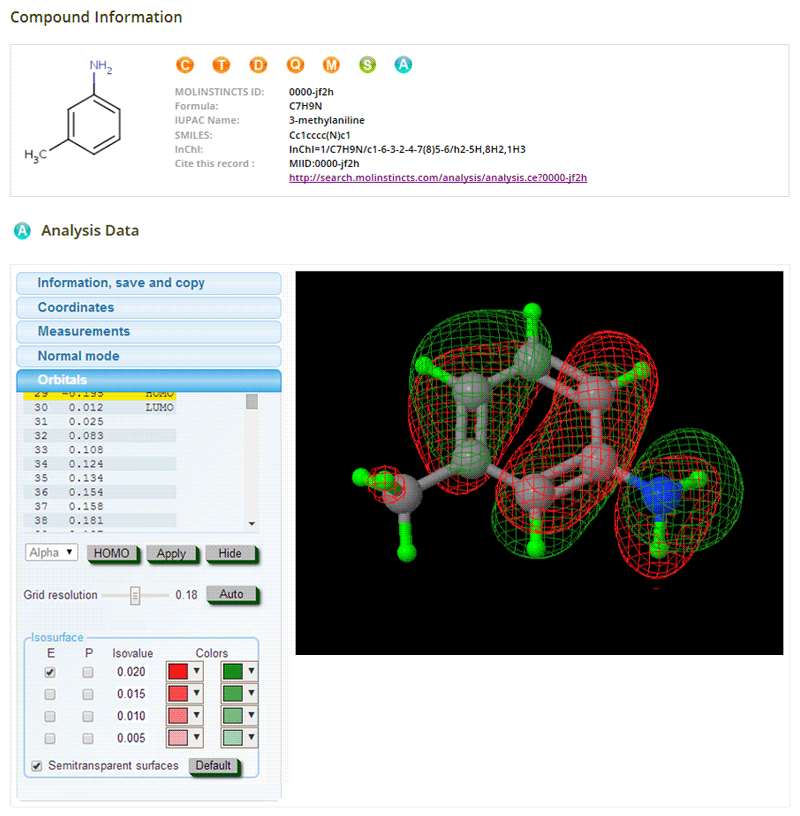
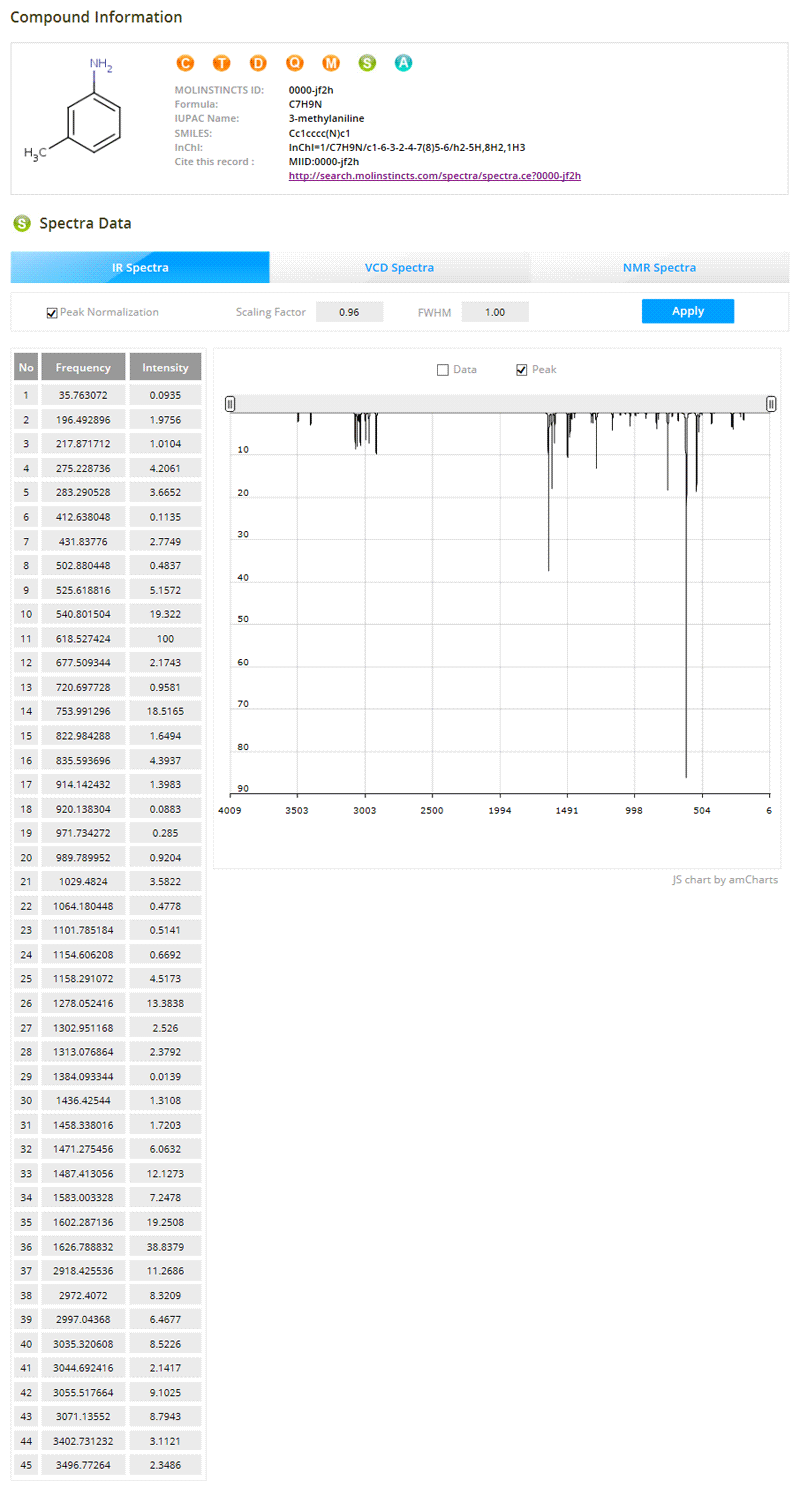
Analytical Information
04- Optimized 3D Structures
- Vibrational Frequency Analysis & Animation
- IR Spectra, VCD Spectra, NMR Spectra
- 2D & 3D Molecular Orbitals
More than 5 million chemical compounds
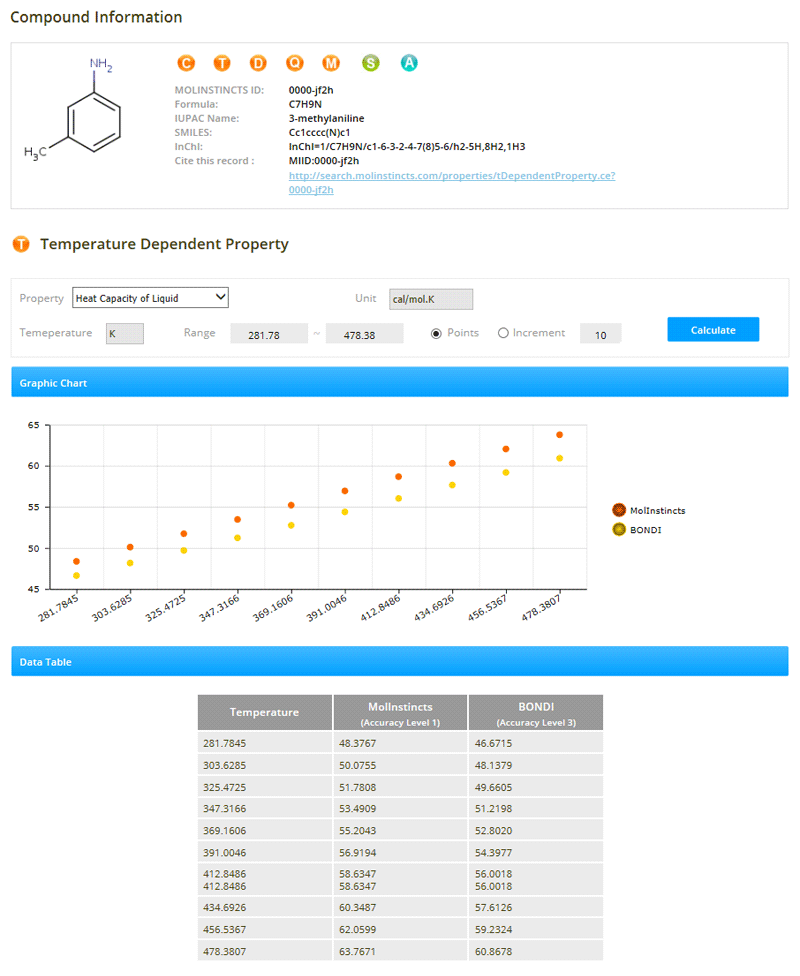
Thermo-Physico-Chemical Properties (temperature-dependent)
02- Heat Capacity of Ideal Gas
- Heat Capacity of Liquid
- Heat of Vaporization
- Liquid Density
- Surface Tension
- Thermal Conductivity of Liquid
- Thermal Conductivity of Gas
- Vapor Pressure of Liquid
- Viscosity of Liquid
- Viscosity of Gas
- Second Virial Coefficient
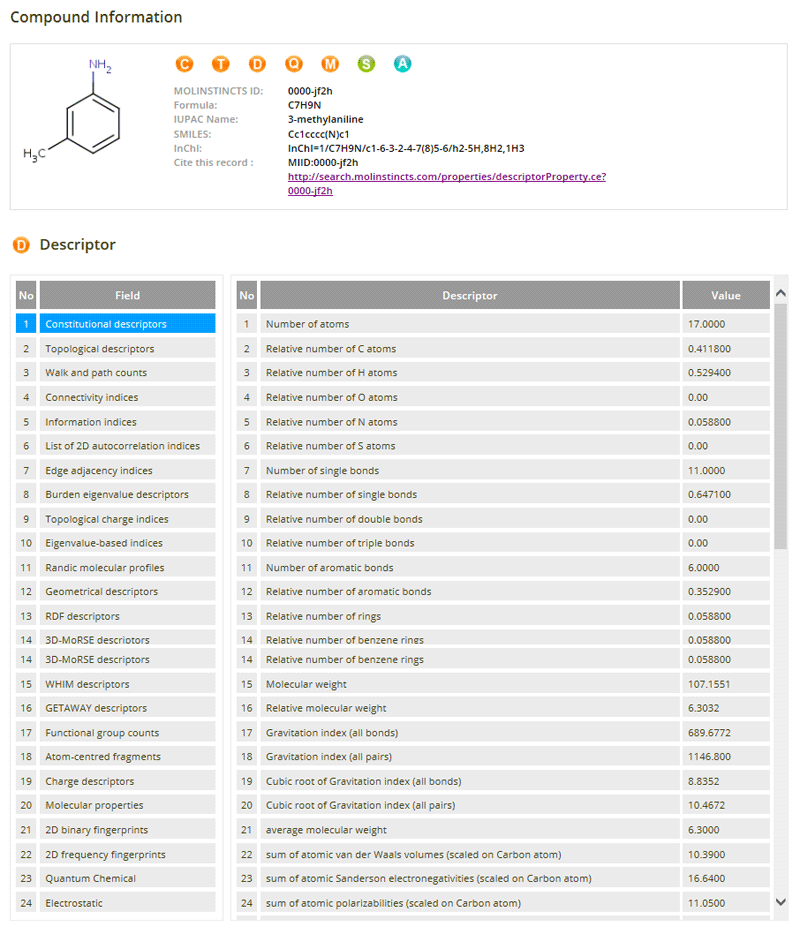
Molecular Descriptors
05- 58Constitutional Descriptors
- 114 Topological Descriptors
- 47 Walk and Path Counts
- 33 Connectivity Indices
- 47 Information Indices
- 96 2D Autocorrelations
- 107 Edge Adjacency Indices
- 64 Burden Eigenvalues Descriptors
- 21 Topological Charge Indices
- 44 Eigenvalue-Based Indices
- 41 Randic Molecular Profiles
- 56 Geometrical Descriptors
- 150 RDF Descriptors
- 160 3D-MoRSE Descriptors
- 99 WHIM Descriptors
- 197 GETAWAY Descriptors
- 130 Functional Group Counts
- 80 Atom-Centred Fragments
- 14 Charge Descriptors
- 26 Molecular Properties
- 100 2D Binary Fingerprints
- 100 2D Frequency Fingerprints
- 65 Electrostatic Descriptors
- 155 Quantum-chemical Descriptors
More than 2,100 sets of data & info per compound
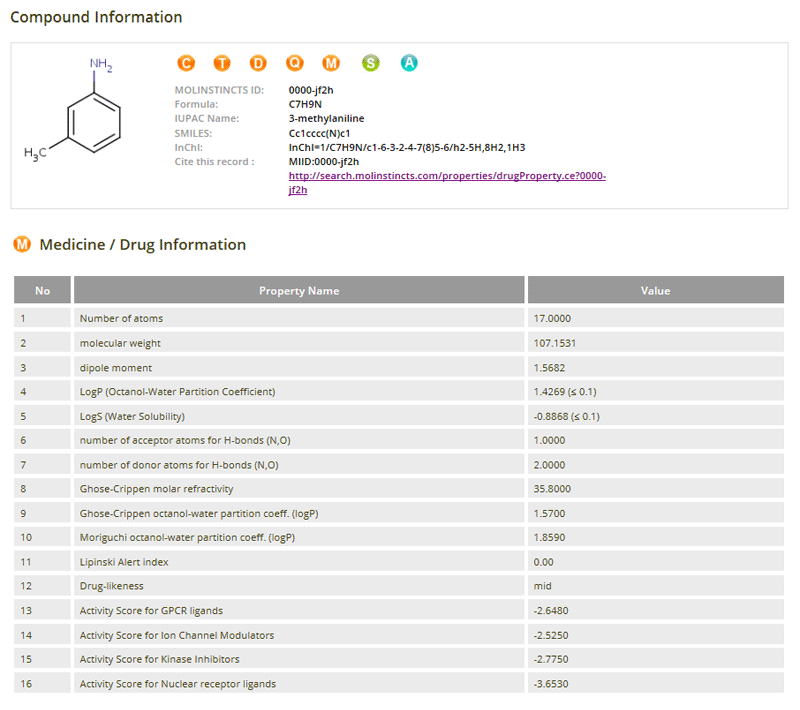
Drug-related Properties
03- LogP (Octanol-Water Partition Coefficient)
- LogS (Water Solubility)
- Number of Acceptor Atoms for H-bonds
(N, O) - Number of Donor Atoms for H-bonds
(N, O) - Ghose-Crippen Molar Refractivity
- Ghose-Crippen Octanol-Water Partition Coefficient (logP)
- Moriguchi Octanol-Water Partition Coefficient (logP)
- Lipinski Alert Index
- Drug-likeness
- Activity Score for GPCR Ligands
- Activity Score for Ion Channel Modulators
- Activity Score for Kinase Inhibitors
- Activity Score for Nuclear Receptor Ligands
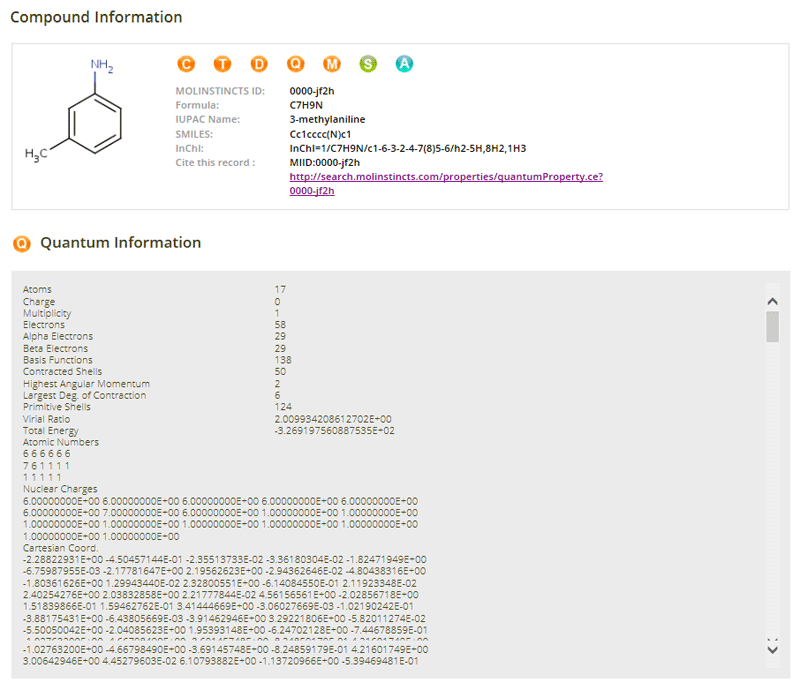
Quantum Properties
06- Atoms
- Charge
- Multiplicity
- Electrons
- Alpha Electrons
- Beta Electrons
- Basis Functions
- Contracted Shells
- Highest Angular Momentum
- Largest Degree of Contraction
- Primitive Shells
- Virial Ratio
- Total Energy
- Atomic Numbers
- Nuclear Charges
- Cartesian Coordinate
- Cartesian Gradient
- Cartesian Force Constants
- Dipole Moment
- Mulliken Charges


 View “Why” Video
View “Why” Video